The Formula for the Conjugate Base of H2c6h6o6 Is
H P O 4 2. Lets cut that to ANY acid and its conjugate base HA.
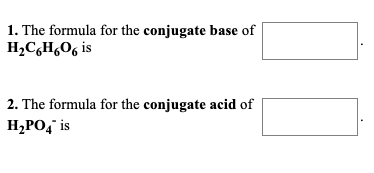
Solved 1 The Formula For The Conjugate Base Of Hch Is 2 Chegg Com
Hence in this reaction we observed that.
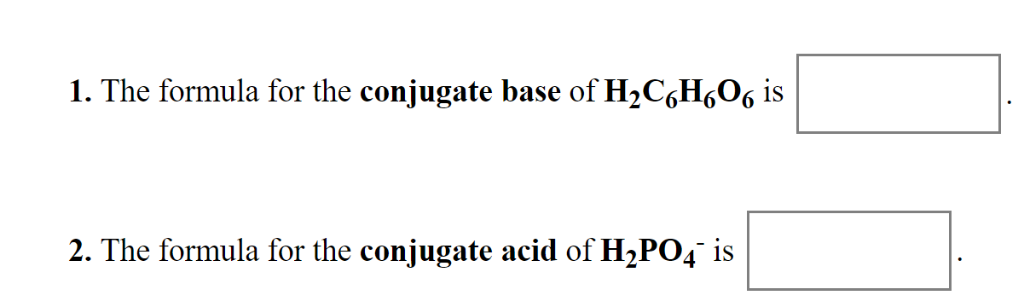
. We review their content and use your feedback to. HNO3aqLi2CO3aqLiNo3aqLiHCO3aq Which component in the net ionic equation is the acid the base the conjugate chemistry A solution that contains 550 g of ascorbic acid Vitamin C in 250. Enter a chemical formula to calculate its molar mass and elemental composition.
We review their content and use your feedback to keep the quality high. Conjugate acid is H2SO4 Conjugate base is SO42 What is The formula for the conjugate base of H2C6H6O6. Identify the acidconjugate base and baseconjugate acid pairs for the following reactions H2CO3aq H2Ol H3Oaq HCO3 -aq C5H5Naq H2Ol C5H5NHaq OH-aq.
The formula for the conjugate acid of HPO42- is. The formula for the conjugate acid of HC6H6O6- is. Who are the experts.
Conjugate acid of C5H5N. Its too long and too confusing using long formulas. The formula for the conjugate base of H2C6H6O6 is___ The formula for the conjugate acid of HS- is_____ arrow_forward.
Chemical formula for lithium hydrogenphosphate with a pH of 97 and conjugate acid when reacted with water Homework Equations Acid Base - Conjugate Base Conjugate Acid The Attempt at a Solution A H2C6H6O6 NaClO - HC6H6O61- NaClO1 I got the acid and conjugate base right but its not taking NaClO or NaOCl as Sodium carbonate. 100 2 ratings Transcribed image text. Conjugate acid of NH3.
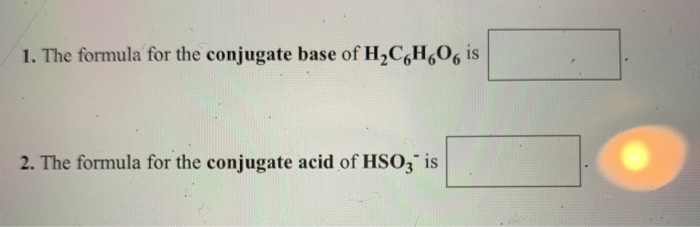
They emit helium gas that attaches to nearby planets. The formula for the conjugate base of H2C6H6O6 is. A sample of a vitamin supplement was analyzed by titrating a 03252 g sample dissolved in water with 00284 M NaOH.
In order to find the conjugate acid of HS- we must first understand the Bronsted Lowery definitions for acids and bases. The formula for the conjugate acid of H2PO4 is. The conjugate base and conjugate acid for HS04 is.
Ascorbic acid vitamin C is a diprotic acid having the formula H2C6H6O6. In the given problem the conjugate base of. If you want this answer you have to get.
A volume of 1327 mL of the base was required to completely. Conjugate acid of C2H5NH2. Molar mass of H2C6H6O6 is 1761241 gmol Convert between H2C6H6O6 weight and moles.
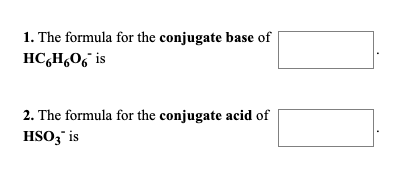
The formula for the conjugate base of H2C6H606 is 2. The conjugate base of the compound is. The formula for the conjugate base of H2C6H6O6 is___ The formula for the conjugate acid of HS- is_____ arrow_forward Give the conjugate acid of the following BrønstedLowry bases.
Experts are tested by Chegg as specialists in their subject area. Write the chemical formula for the conjugate base of each of the following weak acids. Can be recognised with the help of its reaction with water which is.
Write the balanced net ionic equation for the following reaction and determine the conjugate acid-base pairs. When I studied this topic I went into it with this background. Experts are tested by Chegg as specialists in their subject area.
Calculate the OH at 25 C when the H3O 15 x 10 9 M and determine if the solution is acidic basic or neutral. 90 20 ratings Conjugate acid - base pair differ. Who are the experts.
H2C2O4 Explanation- A conjugate base is formed when the species lose an H leaving a ne View the full answer. We review their content and use your feedback to keep the quality high. Practice Write the formula for the conjugate base of the following.
According to the Bronsted-Lowery ac. G of water freezes at 234C. Acid is HX and conjugate base is X-.
1 Answer Hriman Mar 3 2018 SO_3-2 CN- C_2H_3O_2- Explanation. Practice Write the formula for the conjugate acid of the following. H P O 4 2.
Experts are tested by Chegg as specialists in their subject area. Write the formula of the conjugate base of the Brønsted-Lowry acid HCOOH. The conjugate base of an acid is the substance that remains after the acid has donated its protonExample.
Previous question Next question. The conjugate base is A- so if we add that to water we have. Conjugate can be easily recognised in an acid-base reaction as it is an anion.
Acids have one more H ion than there conjugate base pairs so to write a conjugate base pair simply remove a H and subtract a charge -1 HClCl---Since the charge is 0 when we subtract one charge the new charge is -1 or - HCHO2 CHO2---Notice the first H the ionized H was the one that was removed HF F-H2SO3 HSO3-. Bases According to Arrhenius make OH- in water. Conjugate acid of CH3NH2.
The formula for the conjugate base of H 2 C 6 H 6 O 6 is. H P O 4 2 H 2 O P O 4 3 H 3 O.
Solved 1 The Formula For The Conjugate Base Of Hc H20 Is Chegg Com
Solved 1 The Formula For The Conjugate Base Of Hch Is 2 Chegg Com

Solved 1 The Formula For The Conjugate Base Of H2c6h606 Is Chegg Com
No comments for "The Formula for the Conjugate Base of H2c6h6o6 Is"
Post a Comment